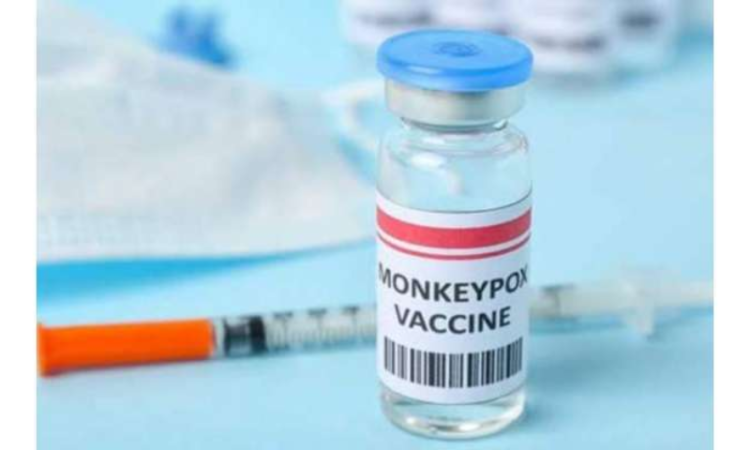
Islamabad, October 14, 2024 – The World Health Organization (WHO) has granted approval for the “M-Pox vaccine” to protect individuals aged 12 to 17 against monkeypox. This decision comes at a time when the European Union had already approved the monkeypox vaccine for adolescents in September.
Additionally, a Danish biotech company is currently preparing to conduct clinical trials this month to evaluate the vaccine’s safety for children aged 2 to 12.
According to media reports, the WHO stated that it approved the pre-qualification of the Jynneos vaccine for teenagers on October 8.
The WHO declared monkeypox a public health emergency for the second time in two years after a new outbreak emerged in the Republic of Congo in August. Last month, the UN agency authorized the first dose of the monkeypox vaccine for adults to ensure its availability in African countries severely affected by the disease.
It is noteworthy that on September 24, the WHO had approved the conditional use of the first monkeypox vaccine, MVA-BN, although it had not been formally licensed for monkeypox. While the WHO deemed the vaccine safe based on preliminary results, it had not officially approved it.
The WHO grants “pre-qualification” approval for vaccines and medicines, allowing countries or regions in need to use them. The MVA-BN vaccine was added to the WHO’s pre-qualified list, meaning that countries and regions could use it at their discretion.